Novosti iz biotehnologije
Inhibition of invadopodia on tumor cells blocks extravasation and metastasis
A new study has shed light on tentacle-like structures called "invadopodia" in cancer metastasis. Genetic and pharmacological inhibition of invadopodia entirely blocked cancer spread by stopping extravasation at endothelial junctions.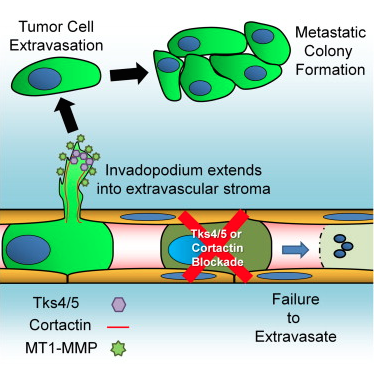
Graphical abstract for the publication: Invadopodia Are Required for Cancer Cell Extravasation and Are a Therapeutic Target for Metastasis, by Leong et, Cell Reports, 2014.
Article summary:
Tumor cell extravasation is a key step during cancer metastasis, yet the precise mechanisms that regulate this dynamic process are unclear. We utilized a high-resolution time-lapse intravital imaging approach to visualize the dynamics of cancer cell extravasation in vivo. During intravascular migration, cancer cells form protrusive structures identified as invadopodia by their enrichment of MT1-MMP, cortactin, Tks4, and importantly Tks5, which localizes exclusively to invadopodia. Cancer cells extend invadopodia through the endothelium into the extravascular stroma prior to their extravasation at endothelial junctions. Genetic or pharmacological inhibition of invadopodia initiation (cortactin), maturation (Tks5), or function (Tks4) resulted in an abrogation of cancer cell extravasation and metastatic colony formation in an experimental mouse lung metastasis model. This provides direct evidence of a functional role for invadopodia during cancer cell extravasation and distant metastasis and reveals an opportunity for therapeutic intervention in this clinically important process.
Article link: http://www.cell.com/cell-reports/abstract/S2211-1247(14)00634-2?_returnURL=http%3A%2%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124714006342%3Fshowall%3Dtrue
Scientists Create Artificial Blood That Can Be Produced On An Industrial Scale
Scientists have found a way to produce human blood, potentially on an industrial scale — thanks to a certain University of Edinburgh professor, Marc Turner, and his program’s funds from the Wellcome Trust.
With this new method, scientists hope they’ll produce a sort of “limitless” supply of type-O red blood cells, free of diseases and able to be transfused into any patient. Blood transfusions are used to replace lost blood after an injury or surgery. According to the National Institutes of Health, every year five million Americans require blood transfusions.
Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C
A nucleotide analog NS5B polymerase inhibitor was approved by the FDA for treatment of chronic hepatitis C (HCV). It is used for genotypes 1-4, in combination with peginterferon and/or ribavirin. Some of the advantages to previous treatments are: activity against all HCV strains, oral administration, fewer adverse effects than peginterferon, and low probability of develping viral resistance.Notably, patients with genotype 1 or 4 HCV infection had a very high rate of sustained virologic response (90%) at 12 weeks.
Some excerpts from the FDA website:
On December 6, 2013, FDA approved SOVALDI (sofosbuvir) tablets for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
SOVALDI is the first drug that has demonstrated safety and efficacy to treat certain types of HCV infection without the need for co-administration of interferon.
SOVALDI is a hepatitis C virus (HCV) nucleotide analog NS5B polymerase inhibitor indicated for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
SOVALDI efficacy has been established in subjects with HCV genotype 1, 2, 3 or 4 infection, including those with hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) and those with HCV/HIV-1 co-infection
DOSAGE AND ADMINISTRATION
The recommended dose of SOVALDI is one 400 mg tablet, taken orally, once daily with or without food.
SOVALDI should be used in combination with ribavirin or in combination with pegylated interferon and ribavirin for the treatment of CHC in adults. The recommended regimen and treatment duration for SOVALDI combination therapy is provided in Table 1.
Table 1 Recommended Regimens and Treatment Duration for SOVALDI Combination Therapy in HCV Mono-infected and HCV/HIV-1 Co-infected Patients
| Treatment | Duration | |
| Patients with genotype 1 or 4 CHC | SOVALDI + peginterferon alfa + ribavirin | 12 weeks |
| Patients with genotype 2 CHC | SOVALDI + ribavirin | 12 weeks |
| Patients with genotype 3 CHC | SOVALDI + ribavirin | 24 weeks |
SOVALDI in combination with ribavirin for 24 weeks can be considered as a therapeutic option for CHC patients with genotype 1 infection who are ineligible to receive an interferon-based regimen.Treatment decision should be guided by an assessment of the potential benefits and risks for the individual patient.
More at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm377920.htm
Links to reported clinical studies: http://www.nejm.org/doi/full/10.1056/NEJMoa1214853#t=article
http://www.nejm.org/doi/full/10.1056/NEJMoa1306218
Neanderthals' genetic legacy: Humans inherited variants affecting disease risk, infertility, skin and hair characteristics
Remnants of Neanderthal DNA in modern humans are associated with genes affecting type 2 diabetes, Crohn's disease, lupus, biliary cirrhosis and smoking behavior. They also concentrate in genes that influence skin and hair characteristics. At the same time, Neanderthal DNA is conspicuously low in regions of the X chromosome and testes-specific genes.
The research, led by Harvard Medical School geneticists and published Jan. 29 in Nature, suggests ways in which genetic material inherited from Neanderthals has proven both adaptive and maladaptive for modern humans. (A related paper by a separate team was published concurrently in Science.)
"Now that we can estimate the probability that a particular genetic variant arose from Neanderthals, we can begin to understand how that inherited DNA affects us," said David Reich, professor of genetics at HMS and senior author of the paper. "We may also learn more about what Neanderthals themselves were like."
In the past few years, studies by groups including Reich's have revealed that present-day people of non-African ancestry trace an average of about 2 percent of their genomes to Neanderthals -- a legacy of interbreeding between humans and Neanderthals that the team previously showed occurred between 40,000 to 80,000 years ago. (Indigenous Africans have little or no Neanderthal DNA because their ancestors did not breed with Neanderthals, who lived in Europe and Asia.)
Several teams have since been able to flag Neanderthal DNA at certain locations in the non-African human genome, but until now, there was no survey of Neanderthal ancestry across the genome and little understanding of the biological significance of that genetic heritage.
"The story of early human evolution is captivating in itself, yet it also has far-reaching implications for understanding the organization of the modern human genome," said Irene A. Eckstrand of the National Institutes of Health's National Institute of General Medical Sciences, which partially funded the research. "Every piece of this story that we uncover tells us more about our ancestors' genetic contributions to modern human health and disease."
More at: http://www.sciencedaily.com/releases/2014/01/140129134956.htm
Original articles: Sriram Sankararaman, Swapan Mallick, Michael Dannemann, Kay Prüfer, Janet Kelso, Svante Pääbo, Nick Patterson, David Reich. The genomic landscape of Neanderthal ancestry in present-day humans. Nature, 2014; DOI: 10.1038/nature12961
New method increases supply of embryonic stem cells
A new method allows for large-scale generation of human embryonic stem cells of high clinical quality. It also allows for production of such cells without destroying any human embryos. The discovery is a big step forward for stem cell research and for the high hopes for replacing damaged cells and thereby curing serious illnesses such as diabetes and Parkinson's disease.
Currently human embryonic stem cells are made from surplus in vitro fertilized (IVF) embryos that are not used for the generation of pregnancies. The embryos do not survive the procedure. Therefore it has been illegal in the USA to to use this method for deriving embryonic stem cell lines. Sweden's legislation has been more permissive. It has been possible to generate embryonic stem cells from excess, early IVF embryos with the permission of the persons donating their eggs and sperm.
An international research team led by Karl Tryggvason, Professor of Medical Chemistry at Karolinska Institutet and Professor at Duke-NUS Graduate Medical School in Singapore has, together with Professor Outi Hovatta at Karolinska Institutet, developed a method that makes it possible to use a single cell from an embryo of eight cells. This embryo can then be re-frozen and, theoretically, be placed in a woman's uterus. The method is already used in Pre-implantation Genetic Diagnosis (PGD) analyses, where a genetic test is carried out on a single cell of an IVF embryo in order to detect potential hereditary diseases. If mutations are are not detected, the embryo is inserted in the woman's uterus, where it can grow into a healthy child.
"We know that an embryo can survive the removal of a single cell. This makes a great ethical difference," says Karl Tryggvason.
The single stem cell is then cultivated on a bed of a human laminin protein known as LN-521 that is normally associated with pluripotent stem cells in the embryo. This allows the stem cell to duplicate and multiply without being contaminated. Previously the cultivation of stem cells has been done on proteins from animals or on human cells, which have contaminated the stem cells through uninhibited production of thousands of proteins.
"We can cultivate the stem cells in a chemically defined, clinical quality environment. This means that one can produce stem cells on a large scale, with the precision required for pharmaceutical production," says Karl Tryggvason.
Embryonic stem cells are pluripotent and can develop into any kind of cell. This means that they can become dopamine producing cells, insulin producing cells, heart muscle cells or eye cells, to name but a few of the hopes placed on cell therapy using stem cells.
"Using this technology the supply of human embryonic stem cells is no longer a problem. It will be possible to establish a bank where stem cells can be matched by tissue type, which is important for avoiding transplants being rejected," says Karl Tryggvason.
Source: http://www.sciencedaily.com/releases/2014/01/140127092934.htm
Original article: Sergey Rodin, Liselotte Antonsson, Colin Niaudet, Oscar E. Simonson, Elina Salmela, Emil M. Hansson, Anna Domogatskaya, Zhijie Xiao, Pauliina Damdimopoulou, Mona Sheikhi, José Inzunza, Ann-Sofie Nilsson, Duncan Baker, Raoul Kuiper, Yi Sun, Elisabeth Blennow, Magnus Nordenskjöld, Karl-Henrik Grinnemo, Juha Kere, Christer Betsholtz, Outi Hovatta, Karl Tryggvason. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nature Communications, 2014; 5 DOI: 10.1038/ncomms4195
Kontakt
Radmile Matejčić 2
51000 Rijeka
Tel. +385 51 584 550
ured@biotech.uniri.hr
OIB: 64218323816
IBAN: HR3724020061400006958
Radmile Matejčić 2, O-047
http://studentska.uniri.hr/
Nadležna studentska liječnica:
dr. sc. Marijana Turčić, dr. med. spec. školske medicine
Radmile Matejčić 5, Rijeka
051/584 875 / 876
e-mail:
skolska.kampus@zzjzpgz.hr












.png)