Biotechnology news
Smart contact lens as a new diagnostic tool
Google, Novartis, California University and partners are developing smart contact lens for diagnostic purposes1. Project was started in 2015, and the clinical studies are expected to be finished in 2016. The idea for the project comes from Sensimed company who first designed sensor contact lens for monitoring intraocular pressure in glaucoma patients2,5. However, this project has a wide range of possible applications in diagnosis and it could be used for measuring blood glucose levels in diabetic patients, correcting presbyopia or detecting environment allergens1,3,4. Also, this contact lens could measure a wide range of carrier's biological data such as body temperature, alcohol levels etc., primarily analyzing physiological characteristics of tears4. Lens could be integrated with smartphones or computers for constant monitoring of physiological markers and data. Project is ecologically friendly since the lens are solar powered.
1. Google, Novartis Smart Lens To Be Ready For Human Trials In 2016 by Jof Enriquez, September 2015.
2. From www.popsci.com, Sensor equipped contact lens monitors glaucoma sympthoms around clock
3. From https://googleblog.blogspot.hr, Introducing our smart contact lens project
4. By Cadie Thompson, October 2015.
5. Piso et al., Modern Monitoring Intraocular Pressure Sensing Devices Based On Application Specific Integrated Circuits, Journal of Biomaterials and Nanobiotechnology, 2012,3,301-309
Inhibition of invadopodia on tumor cells blocks extravasation and metastasis
A new study has shed light on tentacle-like structures called "invadopodia" in cancer metastasis. Genetic and pharmacological inhibition of invadopodia entirely blocked cancer spread by stopping extravasation at endothelial junctions.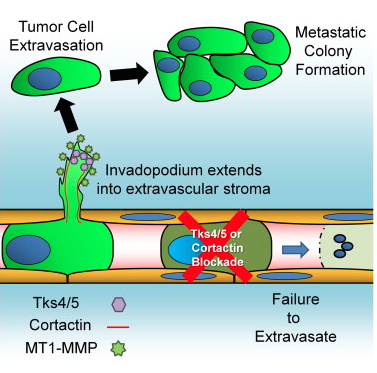
Graphical abstract for the publication: Invadopodia Are Required for Cancer Cell Extravasation and Are a Therapeutic Target for Metastasis, by Leong et, Cell Reports, 2014.
Article summary:
Tumor cell extravasation is a key step during cancer metastasis, yet the precise mechanisms that regulate this dynamic process are unclear. We utilized a high-resolution time-lapse intravital imaging approach to visualize the dynamics of cancer cell extravasation in vivo. During intravascular migration, cancer cells form protrusive structures identified as invadopodia by their enrichment of MT1-MMP, cortactin, Tks4, and importantly Tks5, which localizes exclusively to invadopodia. Cancer cells extend invadopodia through the endothelium into the extravascular stroma prior to their extravasation at endothelial junctions. Genetic or pharmacological inhibition of invadopodia initiation (cortactin), maturation (Tks5), or function (Tks4) resulted in an abrogation of cancer cell extravasation and metastatic colony formation in an experimental mouse lung metastasis model. This provides direct evidence of a functional role for invadopodia during cancer cell extravasation and distant metastasis and reveals an opportunity for therapeutic intervention in this clinically important process.
Article link: http://www.cell.com/cell-reports/abstract/S2211-1247(14)00634-2?_returnURL=http%3A%2%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124714006342%3Fshowall%3Dtrue
Scientists Create Artificial Blood That Can Be Produced On An Industrial Scale
Scientists have found a way to produce human blood, potentially on an industrial scale — thanks to a certain University of Edinburgh professor, Marc Turner, and his program’s funds from the Wellcome Trust.
With this new method, scientists hope they’ll produce a sort of “limitless” supply of type-O red blood cells, free of diseases and able to be transfused into any patient. Blood transfusions are used to replace lost blood after an injury or surgery. According to the National Institutes of Health, every year five million Americans require blood transfusions.
Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C
A nucleotide analog NS5B polymerase inhibitor was approved by the FDA for treatment of chronic hepatitis C (HCV). It is used for genotypes 1-4, in combination with peginterferon and/or ribavirin. Some of the advantages to previous treatments are: activity against all HCV strains, oral administration, fewer adverse effects than peginterferon, and low probability of develping viral resistance.Notably, patients with genotype 1 or 4 HCV infection had a very high rate of sustained virologic response (90%) at 12 weeks.
Some excerpts from the FDA website:
On December 6, 2013, FDA approved SOVALDI (sofosbuvir) tablets for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
SOVALDI is the first drug that has demonstrated safety and efficacy to treat certain types of HCV infection without the need for co-administration of interferon.
SOVALDI is a hepatitis C virus (HCV) nucleotide analog NS5B polymerase inhibitor indicated for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
SOVALDI efficacy has been established in subjects with HCV genotype 1, 2, 3 or 4 infection, including those with hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) and those with HCV/HIV-1 co-infection
DOSAGE AND ADMINISTRATION
The recommended dose of SOVALDI is one 400 mg tablet, taken orally, once daily with or without food.
SOVALDI should be used in combination with ribavirin or in combination with pegylated interferon and ribavirin for the treatment of CHC in adults. The recommended regimen and treatment duration for SOVALDI combination therapy is provided in Table 1.
Table 1 Recommended Regimens and Treatment Duration for SOVALDI Combination Therapy in HCV Mono-infected and HCV/HIV-1 Co-infected Patients
| Treatment | Duration | |
| Patients with genotype 1 or 4 CHC | SOVALDI + peginterferon alfa + ribavirin | 12 weeks |
| Patients with genotype 2 CHC | SOVALDI + ribavirin | 12 weeks |
| Patients with genotype 3 CHC | SOVALDI + ribavirin | 24 weeks |
SOVALDI in combination with ribavirin for 24 weeks can be considered as a therapeutic option for CHC patients with genotype 1 infection who are ineligible to receive an interferon-based regimen.Treatment decision should be guided by an assessment of the potential benefits and risks for the individual patient.
More at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm377920.htm
Links to reported clinical studies: http://www.nejm.org/doi/full/10.1056/NEJMoa1214853#t=article
http://www.nejm.org/doi/full/10.1056/NEJMoa1306218
Neanderthals' genetic legacy: Humans inherited variants affecting disease risk, infertility, skin and hair characteristics
Remnants of Neanderthal DNA in modern humans are associated with genes affecting type 2 diabetes, Crohn's disease, lupus, biliary cirrhosis and smoking behavior. They also concentrate in genes that influence skin and hair characteristics. At the same time, Neanderthal DNA is conspicuously low in regions of the X chromosome and testes-specific genes.
The research, led by Harvard Medical School geneticists and published Jan. 29 in Nature, suggests ways in which genetic material inherited from Neanderthals has proven both adaptive and maladaptive for modern humans. (A related paper by a separate team was published concurrently in Science.)
"Now that we can estimate the probability that a particular genetic variant arose from Neanderthals, we can begin to understand how that inherited DNA affects us," said David Reich, professor of genetics at HMS and senior author of the paper. "We may also learn more about what Neanderthals themselves were like."
In the past few years, studies by groups including Reich's have revealed that present-day people of non-African ancestry trace an average of about 2 percent of their genomes to Neanderthals -- a legacy of interbreeding between humans and Neanderthals that the team previously showed occurred between 40,000 to 80,000 years ago. (Indigenous Africans have little or no Neanderthal DNA because their ancestors did not breed with Neanderthals, who lived in Europe and Asia.)
Several teams have since been able to flag Neanderthal DNA at certain locations in the non-African human genome, but until now, there was no survey of Neanderthal ancestry across the genome and little understanding of the biological significance of that genetic heritage.
"The story of early human evolution is captivating in itself, yet it also has far-reaching implications for understanding the organization of the modern human genome," said Irene A. Eckstrand of the National Institutes of Health's National Institute of General Medical Sciences, which partially funded the research. "Every piece of this story that we uncover tells us more about our ancestors' genetic contributions to modern human health and disease."
More at: http://www.sciencedaily.com/releases/2014/01/140129134956.htm
Original articles: Sriram Sankararaman, Swapan Mallick, Michael Dannemann, Kay Prüfer, Janet Kelso, Svante Pääbo, Nick Patterson, David Reich. The genomic landscape of Neanderthal ancestry in present-day humans. Nature, 2014; DOI: 10.1038/nature12961
Contact
Radmile Matejčić 2
51000 Rijeka
Croatia
Tel. +385 51 584 550
ured@biotech.uniri.hr
OIB (VAT): 64218323816
IBAN: HR3724020061400006958
Radmile Matejčić 2, O-047
http://studentska.uniri.hr/
Biotechnology for the Life Sciences e-mail :














